Our research fields are organic structural chemistry and medicinal chemistry. We analyze the structural properties of molecules, and apply them to design of novel bioactive molecules. Especially, we focused on the aromatic amide and related molecules.
1.Aromatic Architecture Based on the Conformational Properties of Amide and Related Functional Groups
Amide bond is one of significant functional groups for development of bioactive molecules and functional molecules in the field of molecular recognition, supramolecular chemistry and catalysis. Amide and related functional groups such as urea, amidine and guanidine are generally used as building blocks for molecular construction, based on their electronic properties, while the conformational properties of these functional groups with partial double bond character often cause the three-dimensional structures and functions of molecules. Our group found the cis conformational preference of aromatic N-methylated amides. Thus, aromatic secondary amides such as benzanilide exist in trans form both in the crystal and in solution, while their N-methylated amides exist in cis form and predominantly in cis form in solution. This is generic property for aromatic amide, thiamide, amidine, urea, thiorea and guanidine (Fig. 1).1) Further, we applied this cis conformational property to develop aromatic molecules with unique conformations and dynamic behaviors, such as helical molecules and aromatic multilayers, and functional molecules such as asymmetric catalysis and liquid crystal.
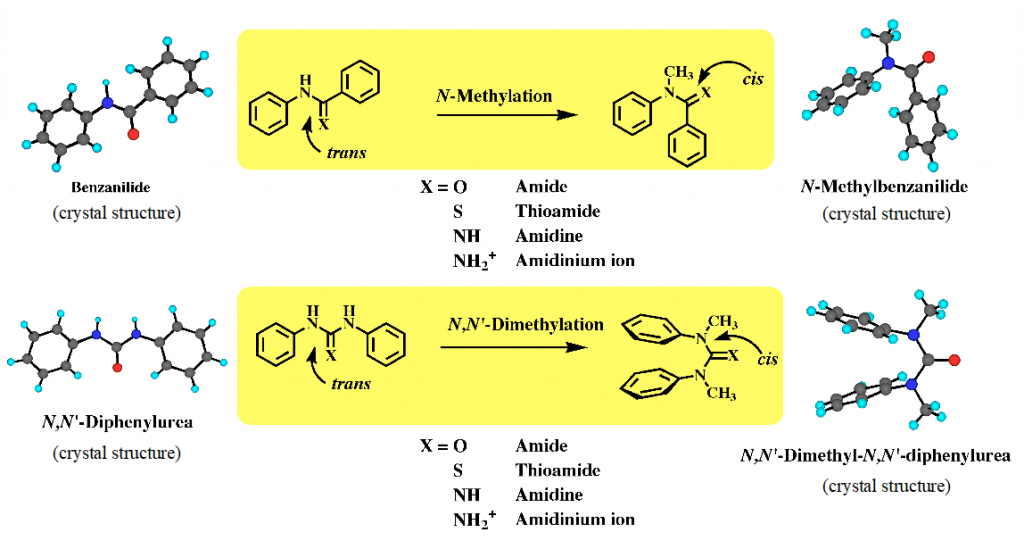
1) Tanatani, A. et al. J. Synth. Org. Chem., Jpn 2000, 58, 556-567.
1)Construction of aromatic multilayers and dynamic helical molecules
In the (cis, cis) structures of aromatic ureas and guanidines, two aromatic rings located at face-to-face position. We developed aromatic multilayered structures by connecting several aromatic rings by N,N’-dimethylated ureas or guanidines (Fig, 2).2) Interestingly, aromatic multilayered guanidines have high water-solubility and high binding affinity to minor grooves of double helical DNAs. Further, meta-linked oligomers exist in dynamic helical structures with all-R or all-S axis-chirality.3,4)
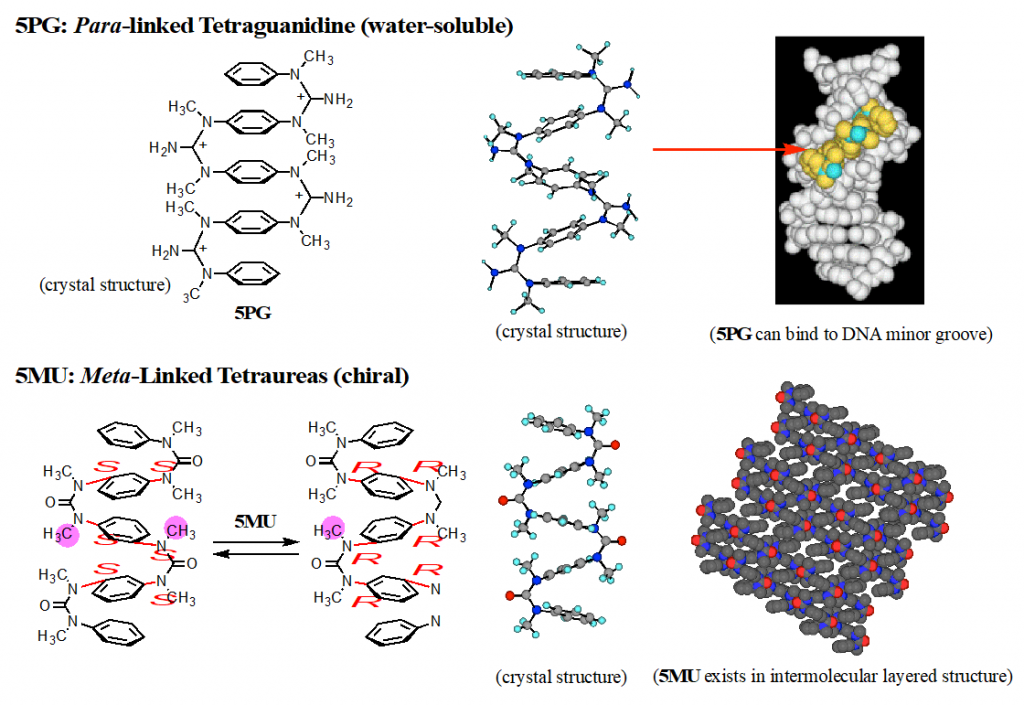
2) Tanatani, A. et al. J. Am. Chem. Soc. 1998, 120, 6433-6442.
3) Kudo, M. et al. J. Org. Chem. 2009, 74, 8154-8163.
4) Kudo, M.; Tanatani, A. New J. Chem. 2015, 39, 3190-3196.
2)Development of Amide Foldamers with Various Aromatic Rings
We have shown that oligomers and polymers of N-methylbenzanilide with cis conformation adapts dynamic helical property. The detailed empirical and theoretical studies revealed the absolute structures of polyamides with chiral N-substituents (Fig 3).5)
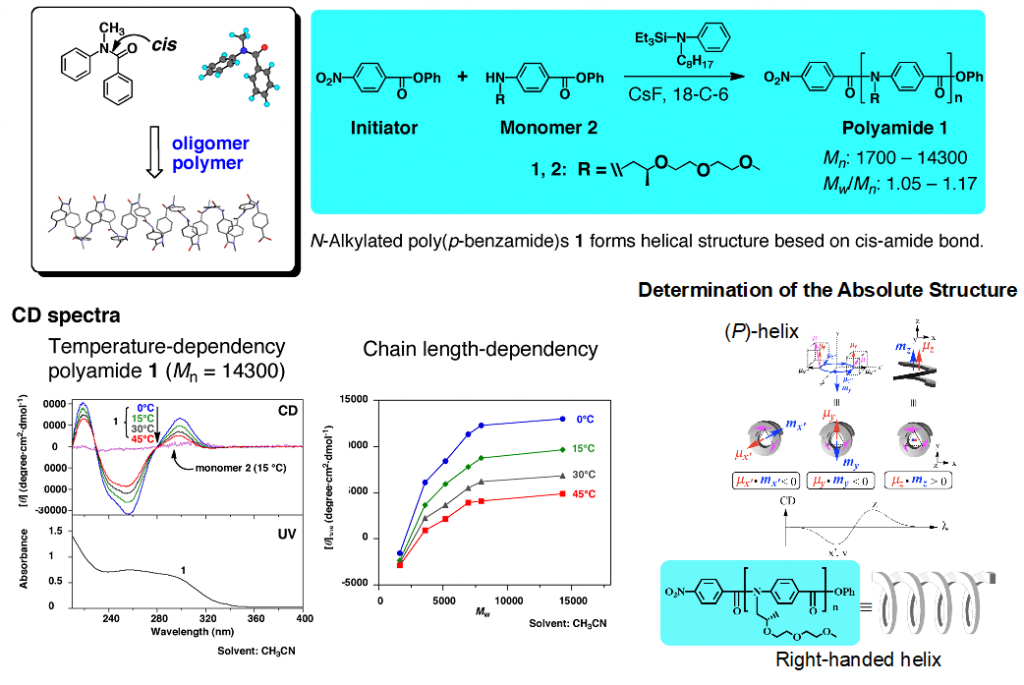
5)Tanatani, A. et al. J. Am. Chem. Soc. 2005, 127, 8553-8561.
Similar folding structures were observed in oligoamides with heterocycles as aromatic rings. For example, the oligoamides in which pyrrole and benzene rings were connected alternately connected by N-alkylated amide bonds existed in helical structures (Fig. 4).6)
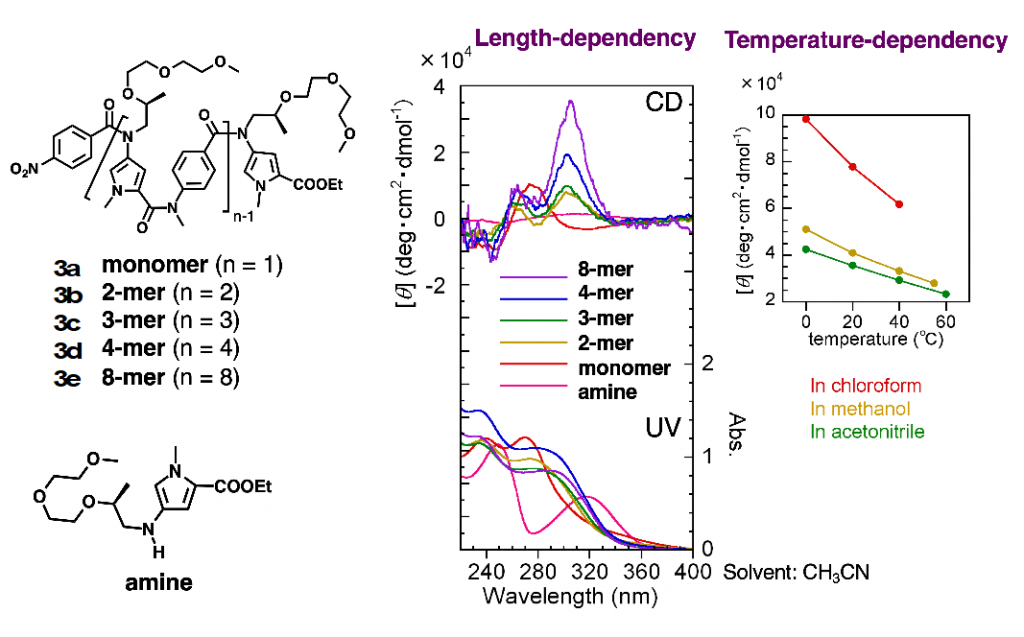
6)Tojo, Y. et al. J. Org. Chem. 2018, 83, 4606-4617.
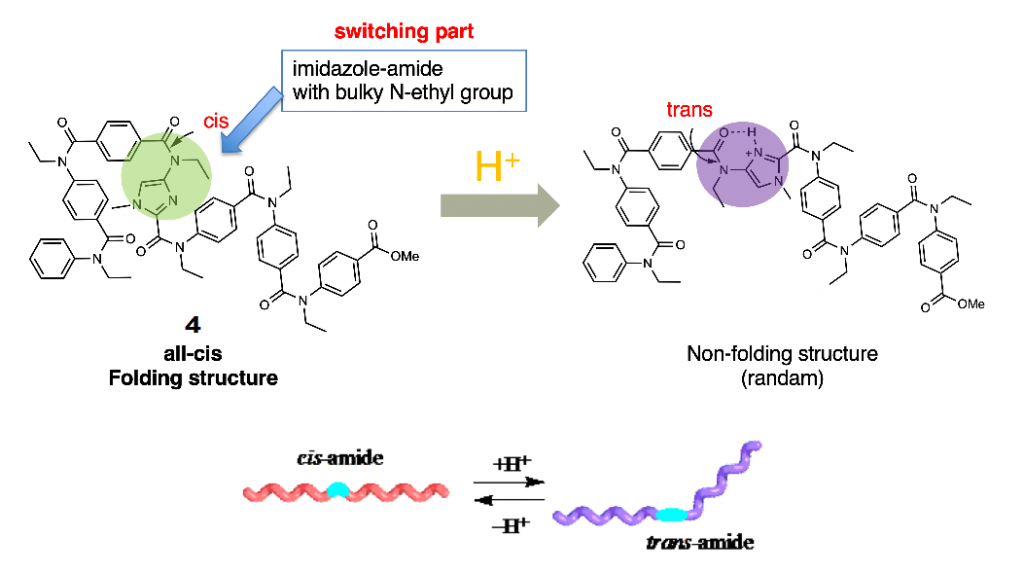
Further, the oligoamides bearing imidazole rings also exist in helical structures, and the addition of acid into the solution of the oligomer caused the conformational alteration from cis structure of amide bond on the imidazole ring to trans structure. This property can be used to development of oligoamides with acid-induced conformational change from well-ordered folded structure to non-folding randam structure (Fig. 5).
3)Development of Asymmetric Catalysis bearing aromatic layered structures with dynamic helical property
We try to apply the chiral property of aromatic layered structure of cis conformational ureas to develop novel asymmetric catalysis. For example, we showed that compound 1 with chiral substituent acted as catalysis for asymmetric aza-henry reaction (Fig. 6).
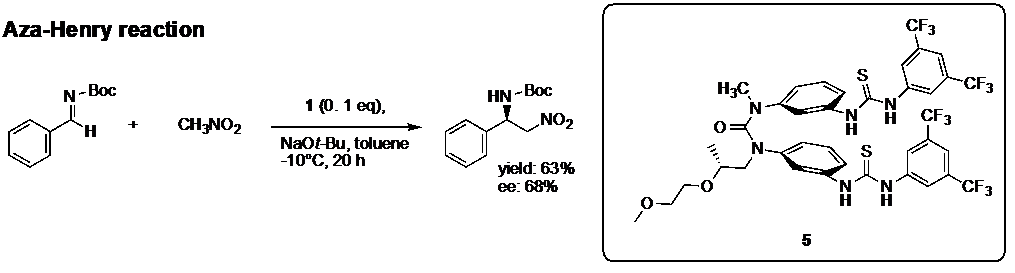
4)Development of Aromatic Molecules Based on the Chemical Properties of Squaramide
Squaramide is an amino derivative of squaric acid bearing a four-membered cyclic structure, and its C–N bond has partial double bond character, like amide bond. In the field of medicinal chemistry, squaramide is regarded as a bioisoster of urea or cyanoguanidine. We have constructed unique aromatic molecules by using squaramide as key building block, such as helical molecules,7) aromatic multi-layered structures,8) molecular switch that altered the conformation in response to the environmental change, and liquid ccrystals9) (Fig. 8).
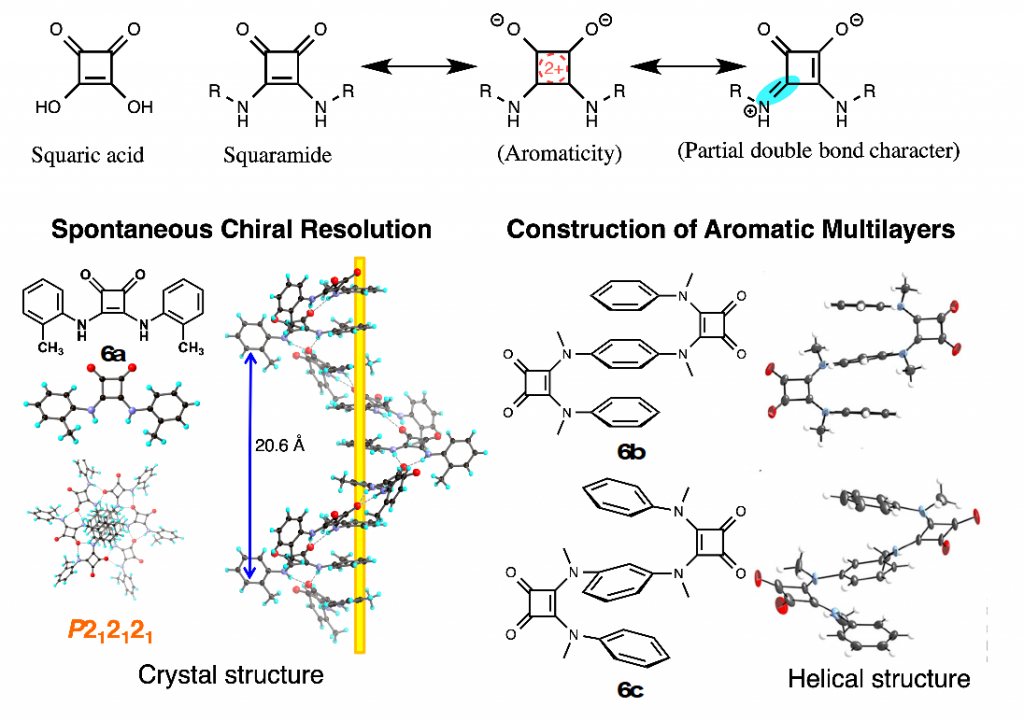
7) Kanda, M. et al. Tetrahedron. 2019, 75, 2771-2777.
8) Arimura, M. et al. Chem. Plus Chem. 2021, 86, 198-205.
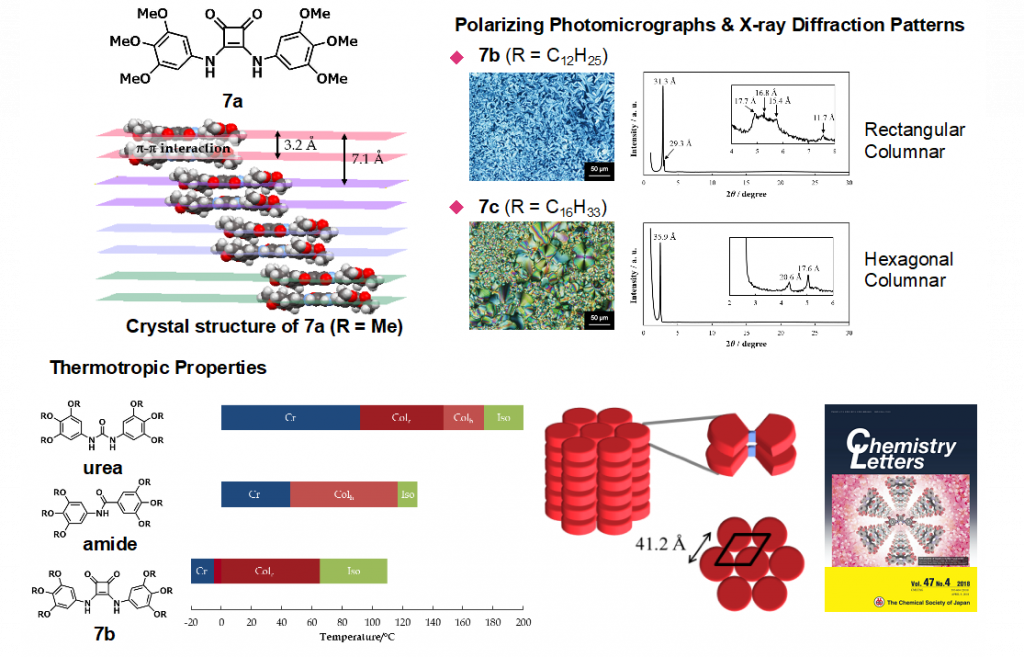
9)Park, S. et al. Chem. Lett. 2018, 47, 601-604
2.Medicinal Chemistry of Nuclear Receptors
Nuclear receptors are ligand-inducible transcriptional factors, responsible for the biological functions of hydrophobic signalling molecules such as steroid hormones and active vitamin A/D. Nuclear receptors are regarded as the molecular targets for drug discovery in the field of cancer, bone diseases, autoimmnune diseases, neurodegenerative diseases, and so on. We develop novel nuclear receptor ligands, especially by applying the structural properties of aromatic amide and related compounds.
1)Development of Androgen Receptor (AR) and Progesterone Receptor (PR) Antagonists
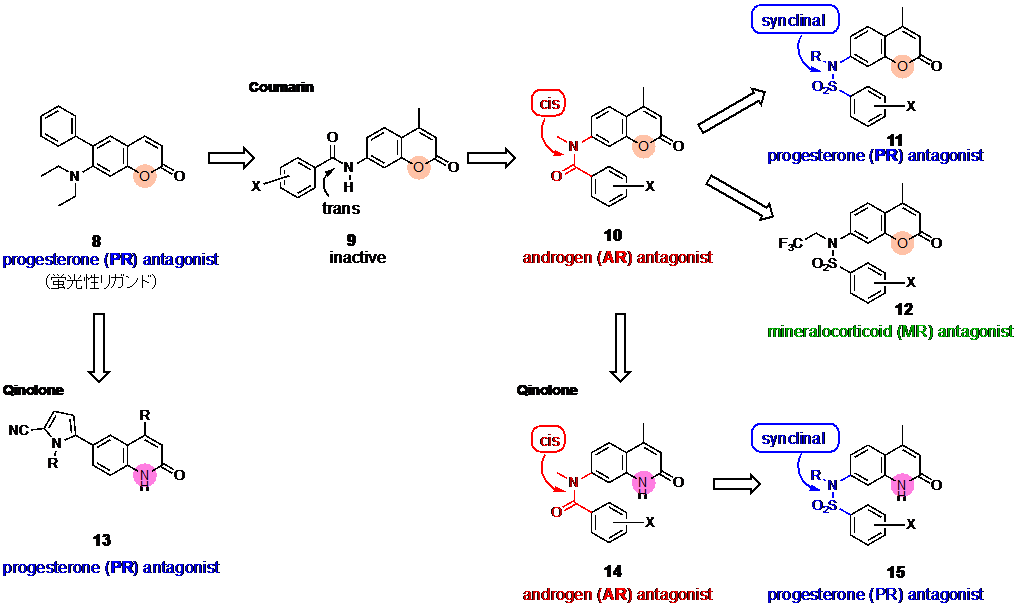
We have developed steroid hormone receptor ligands without steroid skeleton. Previously we designed coumarin derivatives as PR antagonist candidates with unique fluorescent property. Then, we found the compound 1 (Fig. 9) as a potent PR antagonist, and compound 1 exhibited strong fluorescence only when it bound to PR10). By further structural modification, we developed coumarin derivatives 3 with cis-amide structure as AR antagonists11), and compounds 4 with sulfonamide structure as PR antagonists. Thus, the conformation of the linking group changed the target receptor of the molecules. Recently, we found that the replacement of coumarin ring with quinolone ring increased the potency of AR and PR antagonistic activities.
10) Sakai, H. et al. J. Med. Chem., 2011, 54, 7055-7065.
11) Koga, H. et al. Int. J. Mol. Sci., 2020, 21, 5584.
2)Development of Potent Non-secosteroidal Vitamin D Derivatives by Using Lithocholic Acid as A Lead Compound
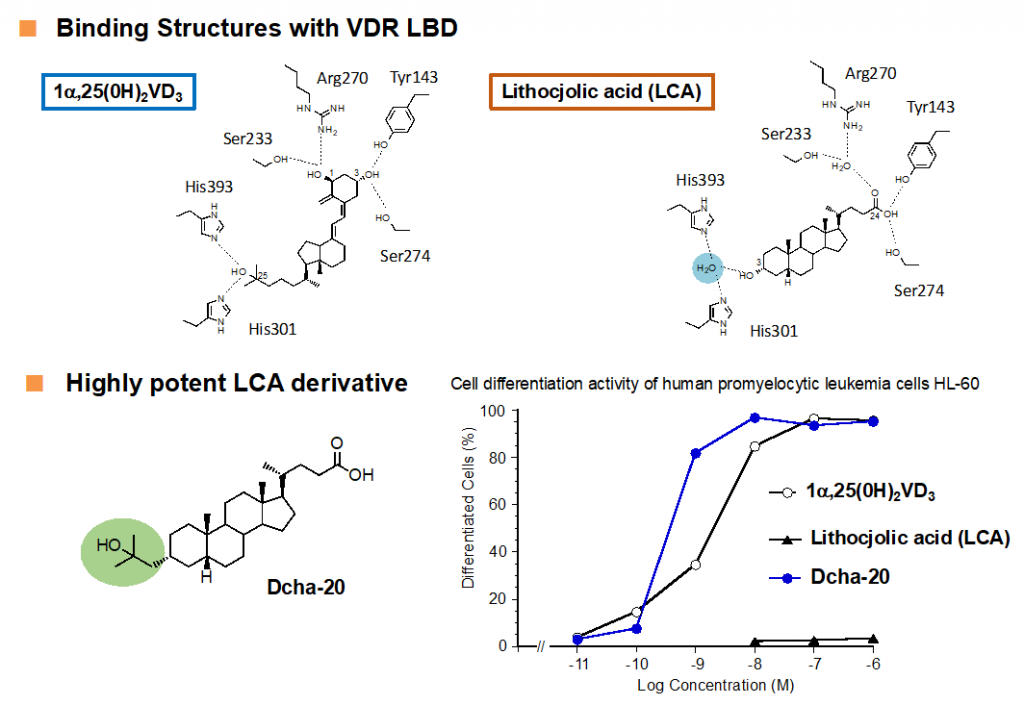
Vitamin D was metabolically activated to afford 1α,25(OH)2D3, which regulates various biological phenomena, including calcium and phosphate homeostasis, bone metabolism, and immune regulation, via binding to and activating vitamin D nuclear receptor (VDR). Various vitamin D derivatives have been developed so far as candidate drugs for skin and bone diseases, while most of potent derivatives have the same secosteroid structure as 1α,25(OH)2D3.n Recently, a few potent non-secosteroidal derivatives have been reported, including our compounds, which are still limited. In 2002, lithocholic acid was reported to be the second endogenous agonist of VDR, but the vitamin D activities of lithocholic acid are very weak, compared to those of 1α,25(OH)2D3. Based on the binding feature of lithocholic acid with VDR deduced by X-ray crystallographic analysis, we developed a lithocholic acid derivative Dcha-20 with more potent vitamin D activity than 1α,25(OH)2D312). Now, the clinical application and further structural development13) of Dcha-20 are on-going.
12) Sasaki, H. et al. J. Med. Chem. 2021, 64, 516-526.
13) Yoshihara, A. et al. Biomol. 2022, 12, 130.
