Research in the Chikamatsu lab focuses on “mixed-anion oxide thin films”.
Oxides with oxygen ions coordinated around metal cations are well used in memory and energy devices. What happens if some of the oxygens in such oxides are replaced by other anions?
Such a substance is called a “mixed-anion oxide”. A schematic diagram is shown in Figure 1. Oxygen and other anions differ greatly in electronegativity and radius. For these reasons, mixed-anion oxides exhibit interesting physical properties not found in oxides. Examples are shown below.
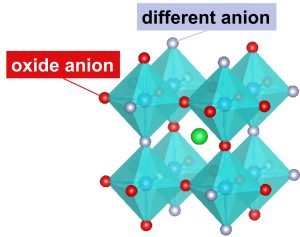
Figure 1. Mixed-anion oxide
・Narrowing the band gap
The energy required for an electron occupying the highest energy orbital to move to the lowest energy empty orbital in a material is called the band gap. In oxides, orbitals are created by the interaction between the orbitals of the metal cations and the orbitals of the oxygens coordinated around them. In general, orbitals with a large metal cation contribution are at higher energies than those with a large oxygen contribution.
By replacing some of the oxygen with anions (such as nitrogen), which have lower electronegativity and higher orbital energy than oxygen, the orbitals with large metal cation contributions and those with large anion contributions are brought closer together. This allows the band gap to narrow.
Replacing some of the oxygen with fluorine can also change the crystal structure. In some cases, the change in environment has changed the way the orbitals hybridize, resulting in a smaller band gap.
One of the physical properties strongly related to the bandgap is photoresponsivity. Photoresponsivity is one of the properties that is currently being actively studied for applications in solar cells and photocatalysts. In both solar cells and photocatalysts, the key to voltage generation and chemical reactions is the transition of electrons due to the absorption of light energy. Since sunlight is used, it is ideal for practical solar cells and photocatalysts to absorb the same energy as sunlight to trigger reactions. However, common metal oxide materials have a bandgap that is larger than the energy equivalent of infrared radiation, making them inefficient at absorbing sunlight, which is the starting point for reactions. If we can reduce the bandgap by making the oxide a mixed anion oxide, we will be closer to realizing a photoresponsive material that can efficiently absorb sunlight.
・Local charge bias
Oxides have a high degree of structural symmetry because the same anions are coordinated around a metal cation. When some of the oxygens are replaced by other anions, the difference in electronegativity causes a difference in electron density, resulting in a polarized electric charge.
Ferroelectricity refers to a material in which spontaneous polarization occurs within the material even without the application of an electric field. An asymmetric structure with locally polarized charge is advantageous for ferroelectricity.
Recently, multiferroic properties related to ferroelectricity have been actively studied. The multiferroic property is a property that combines ferroelectricity and magnetic ordering (orientation of magnetic moments in a material, such as ferromagnetism and antiferromagnetism). Magnetic storage in hard drives, which are all around us, records information as the orientation of the magnetic moment of the storage material. In this process, an electric current must flow to generate the magnetic field that changes the magnetic moment, and the heat generated by the current prevents energy conservation. On the other hand, since there is a correlation between the bias of the electric charge and the direction of the magnetic moment in multiferroic memory, information can be written simply by creating a potential difference at both ends, resulting in energy conservation. However, most multiferroic materials can only exhibit their properties at extremely low temperatures. Therefore, by producing a mixed anion oxide that exhibits ferroelectricity with magnetic order, there is a possibility that it can become a multiferroic material that operates even at room temperature, leading to the development of nonvolatile memory materials with low energy consumption.
・Layered Structure Construction
In mixed anion oxides, the oxygen and other anions do not mix randomly, but are sometimes clearly separated in their occupied positions. In particular, fluoride and hydride (H-) ions have small radii and can enter the interstices in the unit lattice of the parent oxide to form a layered structure.
In layered composite anionic oxides, carrier doping is expected to occur due to anions entering the interlayer, and electron conductivity is expected to occur. In addition, anions with small formal charges can move between the layers by electrochemical reactions. The phenomenon of ions moving through a solid leads to applications in solid electrolytes and neuromorphic devices, which are energy-saving devices that mimic nerve transmission.
As described above, mixed anion oxides have the potential to solve the problems of current oxide materials. So how do they replace oxygen and other anions?
Compared to oxides, mixed anion oxides are unusual materials. This is because the properties of oxygen and other anions are so different that, in most cases, only one of the two anions remains.
To solve this problem, our laboratory has uniquely combined “thin film fabrication technology” and “topochemical reaction technique” to enable the synthesis of novel mixed anion oxides.
・Thin film fabrication technology
Relatively large samples, such as powders and rocks, are called bulk. In contrast, a sample with a thickness of up to 1 µm is called thin film. When other anions are introduced into an oxide, they diffuse through the solid. Because the diffusion rate is faster in a thin film than in the bulk, a thin film sample has an advantage in anion exchange. In addition, by layering thin films of different materials (heterostructure), it is possible to design materials that have the advantages of both materials. Moreover, thin films are obtained in the form of high-quality crystals with few impurities and cracks, making it easier to measure a variety of physical properties. From these perspectives, our laboratory uses a method of preparing oxides in the form of thin films and then introducing other anions into the oxide.
Thin films can be prepared using the pulsed laser deposition (see Facilities page). First, an artificial gemstone plate (substrate), which serves as a nucleus for crystal growth, and a target, which is an object made of sintered powder containing the components of the thin film, are placed inside the equipment so that they face each other. When the target is illuminated with a laser beam, the components are scattered. The scattered components reach the surface of the facing substrate and align with the crystalline order of the substrate. By repeating the laser irradiation for a period of time, the components are stacked, resulting in an oxide thin film.
Normally, the thin film remains attached to the substrate, but it can be peeled off to obtain nanosheets several nm thick.
・Topochemical reaction technique
Topochemical reaction is a low-temperature synthesis method in which an oxide as a precursor and a substance containing an anion to be substituted (anion source) are heated to 500 ºC or lower. The heated anion source produces an anion or a volatile substance containing an anion, which reacts with the oxide to replace the oxygen and the other anion. Topochemical reactions can be used for anion substitution without destroying the structure of the precursor oxide due to their low temperature. In addition, topochemical reactions are kinetically dominated reactions that exploit the activity of volatile anion species, making it easy to obtain metastable phases that would be difficult to synthesize under thermodynamically dominated conditions.
In our laboratory, fluoropolymers are used as fluorine sources for the preparation of oxyfluorides. Fluoropolymers have the advantage of being stable at room temperature and easy to handle.
Recently, we have been working on the preparation of “transition metal oxyfluoride thin films”, in which fluorine is substituted for part of the oxide, and the development of novel physical properties. The radius of fluorine is close to that of oxygen, making it easy to coexist with oxygen even though they have different electrical charges. In addition, fluorine has a small ionic radius, which allows it to enter interstices where other anions cannot, thus producing crystals with a different structure from the precursor. In addition, their small monovalent formal charge allows them to move easily through solids. We are exploiting these advantages to develop materials that exhibit multiferroic properties at room temperature and materials for neuromorphic devices based on the electrochemical insertion/removal of ions into/from solids.
In addition, there are materials in which all the oxygen in the oxide is replaced by fluorine through topochemical reactions. Our goal is to develop fluorine-ion conducting fluorides by this method and to develop materials for the realization of fluorine-ion rechargeable batteries as an alternative to rechargeable batteries using elements such as lithium, which is a scarce resource.
In this way, the Chikamatsu Laboratory aims to discover new properties and functions of oxyfluorides and, based on the knowledge gained, to develop new scientific theories and technologies that will serve as the basis for innovative devices. To elucidate the physical properties of the new thin films and devices we fabricate, we cooperate with many collaborators and actively use state-of-the-art technologies such as synchrotron radiation spectroscopy.
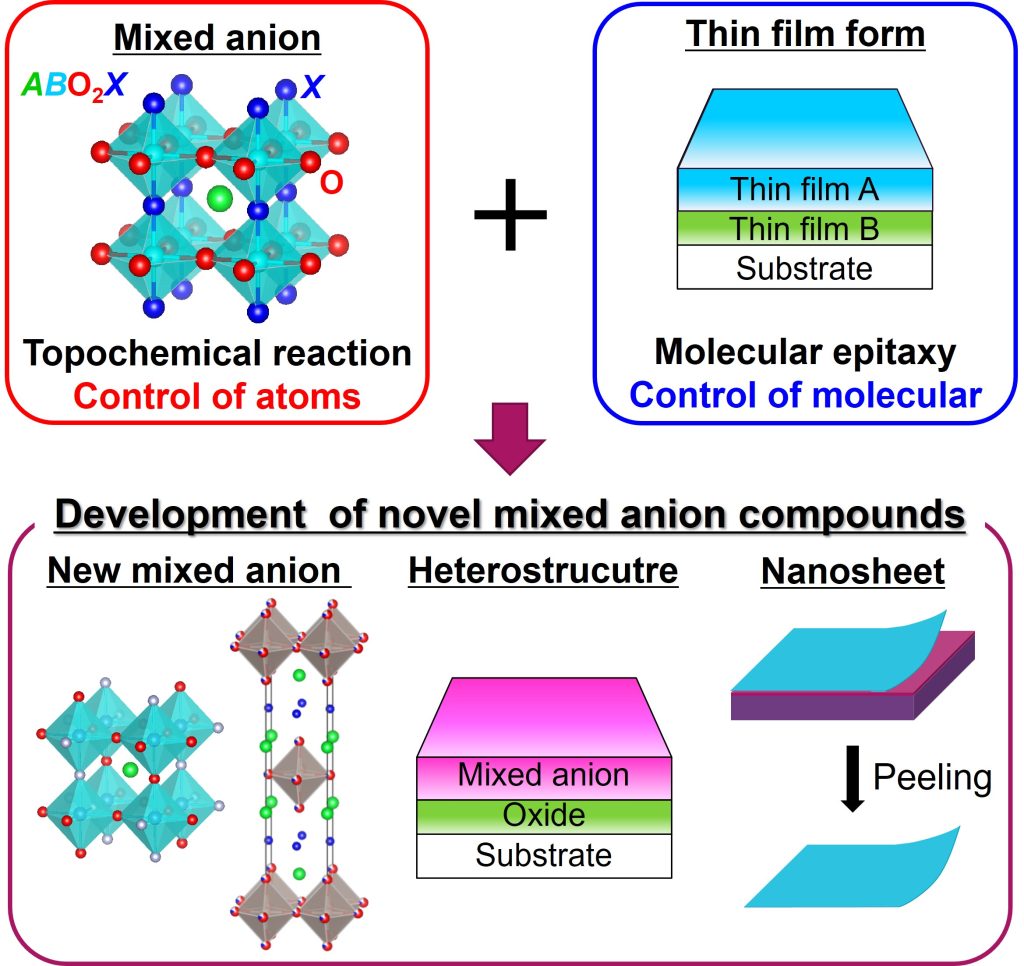
Figure 2. Research in Chikamatsu Laboratory