About us
Our research aims to construct novel metal complexes that enable advanced functional design by taking advantage of the diversity of structures and properties of both metal ligands and metal ions. In particular, we are challenging to create complex systems that work cooperatively by utilizing the structural conversion properties of peptides in crystalline materials (systems that can be called “completely artificial proteins”).
Staff
Ryosuke MIYAKE
Associate Professor
Department of Chemistry, Faculty of Science
Department of Chemistry and Biochemistry, Faculty of Advanced Science, Graduate School of Humanities and Sciences
Reserch
Expansion into huge spaces: Construction of huge ring complexes using flexible skeletons (J. Am. Chem. Soc., 2019)
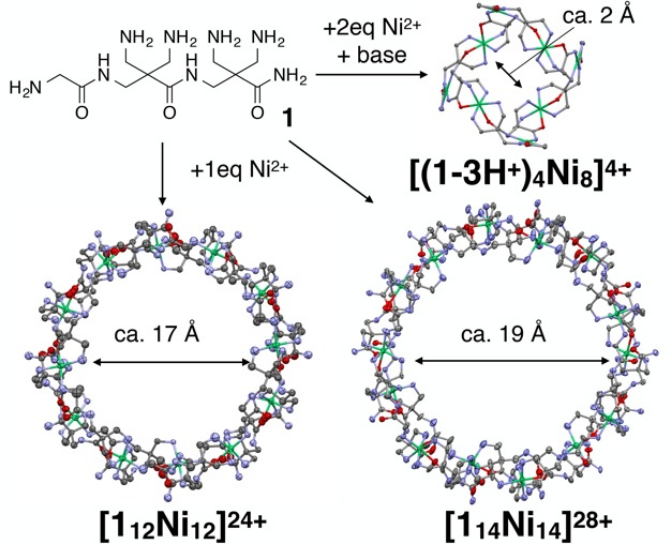
Biological substances such as proteins function by coordinating and controlling the interaction between units through the flexible backbone of their constituent peptides, thereby achieving advanced environmental responsiveness that is difficult to achieve with artificial substances, such as the efficient transport of oxygen molecules by hemoglobin. Among these, the flexible spatial structures created by peptides play an indispensable role in various functions, including precise molecular recognition, efficient substance transport and storage, and protein refolding. Therefore, artificially forming space through flexible skeletons is an important technology for creating advanced functions similar to those found in living organisms. The crystalline space created by peptide cyclic complexes is useful for creating these complex functions, as demonstrated by the research results introduced earlier. However, since spaces made of soft materials are fragile, it has been extremely difficult to assemble large artificial structures (especially those with large spaces) using flexible skeletons.
In this study, based on the idea that even flexible skeletons could form large spaces if stabilized by combining them in a mesh-like structure, we successfully constructed a giant cyclic metal complex with a diameter approaching 20 Å (equivalent to the size of a small protein) using a flexible tripeptide ligand with three types of metal ion-binding sites (see figure below). These tripeptide ligands are thought to link three together via metal ion coordination bonds and can be assembled into a mesh-like structure. In fact, simply mixing the tripeptide ligands and metal ions in solution and crystallizing them allowed us to construct a giant ring-shaped metal complex composed of 14 ligands. Within these structures, the conformation of the tripeptides was determined by metal coordination bonds and hydrogen bonds. Furthermore, due to the flexibility of the backbone, by changing the conditions for forming the metal complex, we were able to construct ring-shaped complexes of various sizes, ranging from those that contracted to one-tenth of their original size to those with slightly different sizes. This result is reminiscent of the characteristic of biological substances that can form diverse shapes of proteins from a limited number of amino acids.
Furthermore, a detailed examination of the crystal structure revealed a complex structure called a catenane, in which giant cyclic complexes formed by 14 ligands were further intertwined with each other. This is the largest catenane structure reported to date. These structures were suggested to exist in solution based on the results of dynamic light scattering measurements and mass spectrometry. Therefore, this study demonstrates the usefulness of this approach in constructing large molecular systems with flexible skeletons (which could be called “completely artificial proteins”).
Creation of temperature-responsive silver two-dimensional arrays utilizing peptide aggregation structures
We have successfully achieved two-dimensional aggregation of silver ions by utilizing the three-dimensional assembly structure of peptide bridge ligands. Due to differences in the interactions between peptide ligands, it is believed that multiple silver ion interactions exist within the two-dimensional array. These silver ion interactions exhibit different temperature responsiveness, and only the distance between silver ions supported by hydrogen bonds between peptides is elongated.
In addition to the research discussed here, we are also engaged in the synthesis and functional evaluation of new compounds that enable structural or property transformations/control in either solid or solution states. We plan to gradually update this information based on papers published in academic journals.
